Differentiating DaxibotulinumtoxinA: An Expert Panel on Early Clinical Experience
Course Description
DaxibotulinumtoxinA, the newest addition to the US BoNTA market, is a new formulation. To assist clinicians in gaining familiarity with and to support confident use of the product in clinical practice, this supplement was developed. Five physicians who have clinical experience with the product met and shared their knowledge, expertise, and practical tips. Their comments were summarized and are presented in this supplement.
To complete this activity and receive credit, the participant should:
- Review the materials on accreditation information, target audience, learning objectives, and disclosure information
- Complete the entire self-study
- Complete the post-test and evaluation/claim form
Educational Objectives
Upon completion of this program, participants should be able to:
- Discuss the basic science of BoNTA products currently approved in the US.
- Understand the proposed mechanism of action for DaxibotulinumtoxinA, thought to underpin the observed longevity in clinical trials.
- Discuss clinical differentiation of BoNTA products.
- Describe any differences in injection techniques for DaxibotulinumtoxinA that have been adopted thus far, in clinical practice.
Commercial Support Acknowledgement
Support for this educational activity is provided by Revance Therapeutics.
Target Audience
This CME-certified activity is primarily intended and designed to educate physicians and extenders who inject botulinumtoxinA products.
Joint Providership
Accreditation Statement
In support of improving patient care, this activity has been planned and implemented by Medical Education Resources (MER) and xMedica. MER is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Credit
Medical Education Resources designates this live activity for a maximum of 1.0 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Academy of Nurse Practitioners (AANP)
The American Academy of Nurse Practitioners (AANP) Certification Board recognizes and accepts continuing education (CE) 1.0 contact hours from activities approved by AMA, ACCME, ANCC, AANP, AAFP and AACN.
Nursing Credit
Medical Education Resources designates this live activity for a maximum of 1.0 ANCC nursing contact hours. Nurses will be awarded contact hours upon successful completion of the activity.
Medical Education Resources is a provider of continuing nursing education by the California Board of Registered Nursing, Provider #CEP 12299, for 1.0 contact hours.
Physician Assistant Credit
Medical Education Resources has been authorized by the American Academy of Physician Assistants (AAPA) to award AAPA Category 1 CME credit for activities planned in accordance with AAPA CME Criteria. This activity is designated for 1.0 AAPA Category 1 CME Credits. Physician Assistants should only claim credit commensurate with the extent of their participation.
Disclosure of Conflicts of Interest
It is the policy of Medical Education Resources to ensure balance, independence, objectivity, and scientific rigor in all of its educational activities. In accordance with this policy, MER identifies conflicts of interest with its instructors, content managers, and other individuals who are in a position to control the content of an activity. Conflicts are resolved by MER to ensure that all scientific research referred to, reported, or used in a continuing education activity conforms to the generally accepted standards of experimental design, data collection, and analysis.
In order to receive credit, please access this link:
https://x-medica.rievent.com/a/41383PrmBSk
Abstract.
Background. The recent introduction of DaxibotulinumtoxinA (DAXI) into the US market represents an exciting addition to the aesthetic treatment armamentarium. For Botulinum toxin (BoNTA) products, differences in formulation give rise to differences in clinical performance, and for DAXI the extended duration of effect and favorable clinical profile observed in clinical studies, even for those patients with severe dynamic lines, is thought to be due to its unique formulation.
Objective. The aim of this manuscript is to provide an overview of BoNTA product differentiation and to discuss the proposed mechanistic differentiators for DAXI. In addition, best practices for incorporating a new BoNTA product in clinical practice are shared.
Methods. As part of a continuing medical education (CME) program, a 2-hour roundtable discussion was held at which the authors, each of whom have experience using DAXI within the context of clinical trials and clinical practice, discussed their early experience.
Results. Thus far, DAXI has been a welcome addition to the aesthetics treatment armamentarium as a potentially longer-lasting toxin with desirable diffusion properties that facilitate natural-looking results. Here, the authors discuss key aspects of DAXI formulation and performance in clinical trials, as well as early real-world experience. DAXI efficacy for severe glabellar lines when targeting the corrugator and procerus muscles is reviewed, as well as field of effect in broader muscles like the frontalis. Best practices for incorporation of a new BoNTA product into clinical practice are reviewed.
Conclusions. With time, clinicians will develop an understanding of the unique attributes of DAXI performance in real-world clinical practice, including performance in off-label areas and level of patient satisfaction.
The introduction of a new technology or product into the aesthetics marketplace brings with it a need to develop a clear picture of real-world performance. While clinical trial data are informative with regard to efficacy and safety, a complete picture of the behavior of a product and its eventual role in clinical practice is undeveloped at the time it first becomes available. For botulinum toxin A (BoNTA) products, differences in clinical performance are largely rooted in manufacturing. Traditionally, these differences in BoNTA manufacturing have given rise to only subtle distinctions; however, for the most recently approved BoNTA product, daxibotulinumtoxinA for injection (DAXI [DAXXIFY™; Revance Nashville, TN]),1 the 6-month median duration of effect in the glabella observed in clinical studies has been a clear product differentiator.2,3 Now that DAXI is commercially available, clinical experience will begin to shape our understanding of DAXI’s unique clinical profile.
The purpose of this manuscript is to provide a picture of early clinical experience with DAXI from physicians who are both experts in BoNTA injection and have clinical trial and real-world experience with DAXI. In addition, a discussion of how DAXI product formulation is thought to be related to the improved durability of treatment effect observed in studies is included. Nuances of DAXI injection are discussed, and best practices for incorporating a new BoNTA product are reviewed.
Methods
As part of a continuing medical education (CME) program on early clinical experience with DAXI, the authors participated in a 2-hour roundtable discussion focused on their early experience using DAXI in clinical practice. Each of the participating physicians is an international expert in BoNTA. The authors served as investigators in DAXI clinical trials, and prior to the meeting, which was held several weeks after regulatory approval by the US Food and Drug Administration (FDA), treated at least 20 patients with DAXI, post-approval. Topics covered included the proposed DAXI mechanism of action, product performance in clinical trials and how these data can inform integration into clinical practice, early real-world experience, and impressions of product differentiation. The authors developed this manuscript to share their early experiences and to help injectors confidently use and assess patient response to a new BoNTA product so that they may optimize their own clinical practice. All patients whose images appear in this manuscript provided written informed consent.
Results
The Biology of Botulinum Toxin Type A
Before discussing how toxin preparations are different from one another, it is important to understand the biology of BoNTA.
Where does BoNTA come from?
In nature, BoNTA is a toxin produced by the soil-dwelling Clostridium botulinum. For humans, BoNTA is of concern because if ingested, for example, through contaminated canned goods (at doses logarithmically higher than used for clinical neuromodulation), it can cause the flaccid paralysis characteristic of botulism.4 This ability of BoNTA to very effectively relax muscles, coupled with the fact that its activity is reversible with time, led physicians as early as 1820 to postulate that BoNTA would be helpful for treating movement disorders and/or spasticity.5 In 1992, the first studies of BoNTA in facial muscles for aesthetic purposes were published.6 By borrowing this powerful protein from nature, and using it in far lower doses as an injection, BoNTA has been developed into not only an important medication for muscle spasticity,7 but also into one of the most sought-after and popular aesthetic treatments in the world for selectively dampening the activity of facial muscles to reduce dynamic lines, adjust brow position, change facial shape, shrink salivary glands, and alleviate the downward pull of depressor muscles in the lower face and neck.8,9
How does the BoNTA protein work?
The signals conveyed by the nervous system to the striated muscles in the face and other parts of the body occur somewhat like a relay race, with the last steps being the release of the neurotransmitter acetylcholine (ACh) from the motor neuron, its passage across the terminal junction between the motor neuron and the muscle fiber, and binding to receptors on the muscle cell, resulting in muscle contraction (Figure 1A).4 When the release of ACh from the motor nerve is blocked by BoNTA, these last steps in the “relay race” are blocked, and muscles no longer contract (Figure 1B). Even if the signal to contract the muscle is sent from the nervous system, ACh is not released from the nerve cell. To stop ACh release, the toxin protein must complete 4 critical steps: 1) bind specifically to the presynaptic membrane of the neuromuscular junction; 2) be internalized into the cell via receptor-medicated endocytosis; 3) undergo rapid escape from the endocytosed vesicle into the cytosol where it can 4) act on its target SNAP-25, thereby preventing release of ACh into the synaptic space.10,11

Figure 1. BoNTA mechanism of action. Normally, acetylcholine (ACh) is released from the motor neuron. When it binds to receptors on the muscle cell, it leads to muscle cell contraction, which can result in the formation of dynamic lines (A). When the release of ACh from the motor nerve is blocked by BoNTA, muscles no longer receive the signal to contract, and the activity of the muscle is modulated (B).
(Image courtesy of xMedica, LLC)
Watch Now
Video 1. Customizing injection with DAXI.
How are BoNTA products manufactured for medical use?
The process of BoNTA manufacturing for medical use requires that the “core” 150 kDa neurotoxin is isolated from the bacterial cells that produce it. Of interest, toxins themselves are not patented, and all available toxin products marketed in the United States include identical toxin proteins.12 Instead, the manufacturing processes employed to generate the individual BoNTA products are patented. Thus, any differences in product behavior are due entirely to manufacturing and formulation. Each manufacturer has a different approach to protein purification and stabilization (Table 1), and it is these differences that give rise to the unique clinical profiles of each product.

Following the purification process, a vial of BoNTA may contain the 150 kDa toxin, complexing proteins (also called accessory proteins, discussed in the sections below), possible cellular contaminants from the bacterial strain that are not removed in the purification process (e.g., other proteins, nucleic acids, cell wall components, etc.), along with buffers and excipients.13
Why is the activity of BoNTA products measured in units? Why can’t I just use the same number of units with each product?
At the end of BoNTA purification, the amount of protein must be quantified. When protein content is measured, it tells us how much product is present. However, just the presence of full-length protein is not a guarantee of functionality.14 This potential disconnect between the amount of protein that is present (expressed as nanograms) and the ability of the protein that is present to function is why the amount of BoNTA in a given vial is expressed as units of activity (U), a measure that takes into account the biological action of a given amount (usually nanograms) of protein. A “unit” of enzyme is the amount of enzyme needed to catalyze a given amount (1 μmol) of substrate in a minute under specified assay conditions. For many biological products, the World Health Organization (WHO) Expert Committee on Biological Standardization defines the assay conditions under which activity must be measured, and all companies that produce that protein/biologic as a drug must use the defined assay so that a “Unit” of product A is the same as a “Unit” of product B. For BoNTA, however, there is no such standard. Instead, each company has its own assay conditions to measure the activity of only the toxin which they produce. The lack of standard experimental conditions makes BoNTA Units completely non-interchangeable and thus, Units of the different toxin products cannot be compared.15 A “Unit” of BoNTA product A is NOT the same as a “Unit” of BoNTA product B. This concept is so important that the FDA requires a statement to this effect to be included in BoNTA prescribing information.1,16,17
Practically speaking, this lack of interchangeability complicates comparison of BoNTA products, especially with respect to clinical data. For example, in studies comparing DAXI to onabotulinumtoxinA (ONA), 40U of DAXI were compared to 20U of ONA.18 While this may seem like a possibly unbalanced comparison, there are 0.18 ng of core neurotoxin present in each of these doses.3,14 Given the lack of interchangeability between units, nanograms of toxin, while imperfect, is one of the few empirical measures that can be used to attempt to assess equivalence.
What are complexing proteins and do they really matter?
In nature, the core toxin is associated with accessory proteins (also referred to as complexing proteins). The genes which code for these accessory proteins are located very close to those for the toxin itself (a gene cluster), and these proteins are expressed together by the bacterial cell.19 Accessory proteins spontaneously associate with the core 150kDa neurotoxin following co-synthesis by the bacteria and the association is mediated by electrostatic interactions.20 These interactions are not covalent bonds, but instead are weaker, charge-based interactions that can be disrupted by relatively small changes in pH or salt concentration in the solution. All toxin preparations with BoNTA molecular weights greater than 150 kDa contain these additional proteins, which make the BoNTA complex “weigh” more (Table 1).
For toxin that is injected for therapeutic purposes, reconstitution with saline and/or the neutral pH of the body causes these proteins to rapidly dissociate,21 and to date, these accessory proteins have not been shown to affect product stability, spread, or durability.22 Overall, their presence does not appear to affect clinical performance. It is important to remember that in nature, the role of BoNTA is one that is part of the Clostridium botulinum bacterial pathogenesis, which is initiated through consumption of contaminated food. This process has been studied extensively, and findings indicate that when ingested, complexing proteins protect the neurotoxin from being destroyed as it passes through the acidic stomach, so that it can reach the neutral pH of the duodenum.23 Once there, the BoNTA accessory proteins disrupt the E-cadherin-mediated intercellular barrier and facilitate absorption of BoNTA through the epithelium and into the circulation, a highly specialized activity which permits pathogenesis.24 Thus, while these complexing proteins are needed to support the pathogenesis of the bacterium in nature, they are not relevant for the action of injected BoNTA.22 In fact, their presence has been linked to an adjuvant effect and thus increased antigenicity and development of neutralizing antibodies,25 which may lead to non-response in some patients.26 Thus, it may be of some benefit to use products lacking these proteins, in order to preserve BoNTA as a therapeutic option for patients later in life or for conditions like migraine.27 The only two available products in the United States that do not contain complexing proteins are IncobotulinumtoxinA and DAXI.
What is an excipient and how is the DAXI excipient different?
In general, an excipient is an inactive component of a given drug that serves to stabilize the active molecule or enzyme. Excipients can have a range of roles, including prevention of aggregation (clumping of proteins that renders them inactive), increased absorption by the body, or serving as a bulking agent for potent active ingredients so that the volume administered is reasonable in clinical practice. For traditional BoNTA products, the excipient used is human serum albumin (HSA), which is used along with buffers and sugars to stabilize the protein and maintain pH. However, the excipient for DAXI is completely unique. Rather than HSA, the manufacturer developed a peptide to serve this stabilizing function: RTP004. RTP004 is a small, 35-amino acid (5 kDa) protein fragment (Figure 2).3 The peptide contains 15 consecutive lysines, which are highly positively charged at physiologic pH and form strong electrostatic bonds with DAXI. On either side of the 15-lysine residues is a 10-amino acid protein transduction domain (PTD) that is also highly positively charged. The domain, like BoNTA itself, is appropriated from another highly efficient natural system. The 10 amino acids are a small fragment of the HIV-TAT protein (the full-length TAT protein is 101 amino acids),28,29 and can be thought of as a cell-penetrating peptide. In the field of molecular biology, the PTD is an extraordinarily valuable and well-studied tool because it can be attached to proteins under study to ensure efficient, unidirectional transport into the cell (the protein can only move into the cell and not out).29 It has been used for intracellular delivery of molecules as varied as gold particles, proteins, and other pharmacologic agents.20

Figure 2. Amino acid sequence of RTP004. The PTD domains are shown in blue, and the poly-lysine (K) sequence is shown in black.
Importantly, the PTD is not thought to have immunogenic potential,29,30 and because RTP004 itself is a synthetic protein, it is not isolated from a cell system but is instead synthesized from individual amino acids in vitro. Importantly, any insinuation that DAXI’s RTP004 peptide is “from HIV” due to the similarity of ten amino acids is incorrect.
As an excipient, RTP004 is highly effective, and only the combination of RTP004 and DAXI’s sugars and surfactants, at the levels present in the DAXI formulation, is optimal to achieve complete protection against surface adsorption of DAXI to vial surfaces in all manufacturing steps.31 RTP004 also prevents aggregation and stabilizes the DAXI toxin in a concentration-dependent manner.32 These advantages are important because they help to ensure that no toxin molecules are lost during product preparation and administration in the clinic.
What do the data tell us about DAXI’s unique clinical features?
For most traditional BoNTA products, differences in manufacturing give rise to subtle clinical differences; however, for DAXI, the effect of formulation on product performance is substantial. Evidence collected from multiple clinical trials including more than 3800 patients consistently demonstrates faster time to onset, higher response rate, and increased duration of effect.2,3
Because patients are most interested in whether a product “works” for them (a high rate of efficacy irrespective of baseline severity) and how durable it is (how long the effect lasts), these findings are exciting for practical clinical reasons. Thus far, the high response rate observed for DAXI (84.6% at week 4; based on at least a 2-point improvement on the Investigator Global Assessment—Facial Wrinkle Severity [IGA-FWS] scale) versus ONA (76.2% at week 4) is consistent with observations in clinical practice, with a reduced need for “touch-up” treatments.33
One additional pattern noted in early clinical use is that patients who have had difficulty obtaining an optimal response in spite of tailored treatment and appropriate dosing with traditional BoNTA products are able to achieve excellent results with DAXI (Figure 3). This observation is consistent with the finding that the proportion of subjects with severe glabellar lines treated with 40U DAXI who achieved a 3-point improvement peaked at 83% at weeks 2 to 4 compared with a peak of 50% at weeks 2 to 4 for ONA 20U.18 It should be noted, however, these comparisons are from different studies and that head-to-head comparisons are needed for a true comparison.

Figure 3. A 28-year-old male at baseline (A) and 2 weeks after treatment with 40U of DAXI in the glabella and frontalis (B). This patient has severe glabellar lines at baseline and was able to obtain an even, natural-looking effect..
(Image courtesy of Steven Fagien, MD, FACS)
BoNTA clinical effect can be measured in a range of ways, including “response rate”, which can be a 1- or 2-point improvement on the selected severity scale, the proportion of subjects with “none” or “mild” glabellar lines on the selected scale, and time to return to baseline, among other metrics. The use of different scales and different primary endpoints in studies makes toxins hard to compare. In addition, the translation of these endpoints to clinical practice can be confusing and difficult. Instead, analysis of study data to determine the time to loss of “none” or “mild” glabellar line severity provides a clinically relevant metric, as this outcome is most closely tied to the optimal time for retreatment. For 40U DAXI, the median time point for maintaining “none” or “mild” glabellar lines is 24 weeks (168 days) while for 20U ONA, the median duration of effect for maintaining “none” or “mild” severity is 120 days.1,34 By definition, at the median time point, half of patients have a shorter duration of treatment and half a longer duration.1 This difference in median time to loss of “none” or “mild” means that patients treated with DAXI might expect to have a longer duration of effect, less frequent injections, and to spend far more time with glabellar lines with a severity of “mild” or “none.” Importantly, this extended duration is for the DAXI registration dose. In contrast, high-dose studies for other toxin products show that doubling or even quadrupling the registration dose results in a modest improvement in duration. Thus far, in clinical practice, using the disconnect between the cost of doubling the amount of product injected and the achievable duration has made use of these doses impractical.35
Though the hazards of comparing clinical trials generally prevent a true comparison of most toxin products, the DAXI dose-ranging studies and Phase 2 studies are an exception, as they include 20U of ONA as a comparator.18,33 This is a unique advantage because it permits clinicians to confidently assess differences in performance. Importantly, these data on duration were generated in the glabellar complex using a prescribed injection pattern for the purposes of garnering regulatory approval, not to optimize real-world use. In the coming months, injectors and patients together will develop a better understanding of longevity in off-label areas, as well as duration of effect when different dilutions and/or injection techniques are used. Thus far, experience with injection of the glabellar complex has been positive (Figure 4).
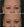
Figure 4. A 51-year-old female at baseline (A), and 7 weeks after treatment with 40 U of DAXI in the glabella (B). Note: patient has significant brow elevation post treatment at rest.
(Image courtesy of Sue Ellen Cox, MD, FAAD)
How might the DAXI formulation allow for longer duration and greater clinical effect?
In nature, ingested BoNTA must pass into the circulation and “find” its target cells, the cholinergic nerve terminals.36 Of course, when BoNTA is injected for therapeutic or cosmetic purposes, the injector places the toxin in the area where diminished muscle contraction is desired, but even then, the BoNTA must “find” the right cells. This is accomplished through a remarkable process that can help us to understand why the RT004 peptide may increase the effect of injected toxin.
All BoNTAs bind to the nerve terminal through a 2-step process that permits selective binding.4 This first step is critically important because it allows BoNTA to bind to the cell even if the toxin is present at tiny concentrations while fluid in the extracellular space is moving rapidly (and can wash away the toxin) and the nerve terminal itself is a tiny target surface.4 This first step relies on binding to glycosphingolipids, negatively charged molecules embedded in the cellular membrane of the nerve terminals. Normally, glycosphingolipids are thought to serve the function of “catching” neurotransmitters as they are released so that they do not diffuse away, but instead can be held on the membrane and routed to their respective high-affinity receptors (or held as a reservoir as receptors are recycled).37 The first binding step for BoNTA is to bind the very distal end of a glycosphingolipid. In this way, BoNTA takes advantage of the natural system that usually works to permit efficient functioning of our own nervous system to accumulate on the cell surface. BoNTA itself is a dipole and contains positively charged amino acids that facilitate this nonspecific binding to the negatively charged nerve terminal; however, the positively charged residues in RTP004 (both the poly-lysines and the PTDs are positively charged) likely further facilitate this initial binding event, increasing the amount of BoNTA that accumulates on the nerve terminals. This hypothesis is supported by findings in preclinical models showing an increased binding affinity for BoNTA + RTP004 to nerve terminals compared to an identical toxin without RTP004.38,39 This initial binding event is rapid and followed by a second binding to the synaptic vesicle receptor SV2, which results in an even tighter binding and receptor-mediated endocytosis of BoNTA into the cell.4
Stated in plain language, the scientific and clinical observations of DAXI BoNTA suggest that it may be “stickier” than products formulated without RTP004. While the toxin itself once inside the cell works the same way as other BoNTA products, DAXI is able to “stick” to the nerves we are trying to treat with BoNTA without increasing the actual amount of BoNTA injected. In other words, the RTP004 peptide is able to increase the local concentration of BoNTA at the nerve terminal by improving accumulation of BoNTA and/or limiting the loss (or random spread) of BoNTA away from the area of injection. This attraction to the nerve terminals may underpin the observation in clinical studies that increased longevity is not associated with increased risk of side effects.2 Thus, DAXI takes advantage of the way that BoNTA binds to its target cells in nature to improve DAXI efficiency.
How could diffusion improve clinical effect?
Before discussing diffusion, it is important to differentiate spread and diffusion, as the two are often conflated. Spread means that injected BoNTA travels from the original injection site and acts on tissues outside of the intended area (think of the way droplets of ink spread in a glass of water).11 Spread can lead to adverse events, and results from suboptimal injection technique, injection of too large a volume, or inappropriate needle size. Diffusion describes the passive movement of BoNTA along a concentration gradient, which can happen within the target tissue. Diffusion gives rise to a product having the greatest effect at the point of injection, but a subtle effect as the product diffuses along a concentration gradient, a natural phenomenon that may improve treatment outcomes. Anecdotally, DAXI appears to act along the muscle fiber, perhaps owing to an increased affinity for cholinergic nerve terminals present along the muscle fibers. Theoretically, a higher affinity would prevent the toxin from moving away from the target tissue (through the extracellular space, where efficacy could be diminished) and instead would give rise to a somewhat wider field of effect within the target muscle. Thus diffusion, in combination with a higher affinity for the nerve terminals, could account for the “smooth and even” results seen with DAXI. Because “skip areas” are a common reason for patients to return for touch-up treatments with other BoNTA products, this effect may emerge as a subtle but important advantage in clinical practice with DAXI. For example, a focused product diffusion from the site of injection along the muscle plane of the orbicularis oculi may account for the reduction in compensatory medial muscle movement avoiding a perceived worsening of inferior eyelid rhytids often seen when treating lateral canthal lines as demonstrated in Figure 5. Additionally, for broad, flat muscles like the frontalis, this diffusion within the target tissue appears to prevent a patchwork-like outcome. In addition, overall, a more uniform denervation could relate to enhanced longevity (personal communication, S. Fagien). For the patient shown in Figure 6, injection in the upper forehead and glabella gives rise to an even, natural-looking effect.

Figure 5. A 52-year-old female at baseline (A), and 7 weeks after treatment with 20 U of DAXI per side in the crow’s feet (B). Note post treatment patient eye aperture is enhanced at expression.
(Image courtesy of Sue Ellen Cox, MD, FAAD)

Figure 6. A 30-year-old female at baseline (A, at rest; B, at maximum frown; and C, at maximum lift) and 12 weeks following treatment with 40U of DAXI in the glabella and 16U in the upper forehead (D, at rest; E, at maximum frown; and F, at maximum lift)
(Image courtesy of Rebecca Fitzgerald, MD)
Importantly, as the frontalis muscle is the primary brow elevator, optimal cosmetic treatment of forehead lines with neuromodulators, irrespective of product used, can be challenging, and may result in dropped brows or lids. This is commonly assumed to be from aggressive dosing or too much product in the lower forehead; however, recent literature provides evidence that this can also be seen when targeting the glabellar complex alone, as the skin insertions of the procerus and corrugators are interdigitated with the frontalis superiorly.40 Thus, injection of BoNTA into the superior aspect of the glabella can act on the fibers of the frontalis and cause medial brow drop and/or unmasking of upper eyelid ptosis.40,41 In patients with pre-existing ptosis, the loss of compensatory action by the frontalis due to an unrecognized levator disinsertion (i.e., pre-existing mild upper eyelid ptosis) can result in overt ptosis. The frontalis is the primary brow elevator, so injections placed too high are more likely to unmask upper eyelid ptosis (personal communication, M. Kane). Our understanding of the anatomy of the muscles of upper facial expression continues to evolve and inform newer/modified neuromodulator injection patterns which optimize patient outcomes and avoid adverse events. For example, a recent retrospective post hoc analysis by Bertucci et al.40 revealed that the best brow outcomes for patients treated with 40U of DAXI in the glabella were achieved with deep injection into the medial corrugator (just below the medial hair bearing brow), deep low midline procerus injections (at the lower medial brow line), and superficial lateral corrugator injections placed between the midpupil and lateral limbus (Figure 7). Overall, glabellar injection techniques that more precisely targeted the corrugator muscles also resulted in improved treatment duration compared to other techniques. Furthermore, medial corrugator injections above the medial brow, procerus injections superior to the inferomedial brow, and lateral corrugator injections administered deeply or more medially, toward the medial third of the brow, were more often associated with suboptimal outcomes more apparent during dynamic expression. By considering the most up-to-date understanding of functional anatomy, both in the upper face and other areas treated with BoNTA products, injectors are more likely to be able to consistently deliver excellent outcomes for their patients.

Figure 7. Injection technique and sites for optimal results described by Bertucci et al.40 The filled circles represent deeper injections, and the open circles are more superficial injections.
What is the best way to dilute DAXI?
In clinical practice, it is important to preserve work flows and minimize the risk of inadvertent injection of too much or too little product. For this reason, the authors reconstitute DAXI so that push volumes used are equal to those used for other toxins in the practice. Reconstitution volumes vary from 1.0 cc to 2.5 cc depending on the practice. Most authors use preserved saline because it improves patient comfort.
What is the best way to incorporate DAXI into clinical practice?
Diligent patient assessment both before and after treatment is a central part of understanding product behavior. In order to develop a clear view of clinical effect, have initial patients (approximately 12-15 patients) return for follow up at 4 weeks to assess effect at 2 weeks post injection. An astute observer can develop a clear understanding of product behavior within a relatively short time. Feedback from the patient can be used to gauge satisfaction and can inform the need for any modifications to injection patterns and/or dose. As with all BoNTA products, an understanding of anatomy (Figure 8) is critical, as well as understanding of how to customize treatment based on patient characteristics like muscle mass, (especially in male patients) or area treated.42 Furthermore, patient needs may change over time as compensatory muscle groups become more active or as muscles that are treated repeatedly begin to atrophy. Understanding how individual anatomy can be inferred through assessment of dynamic line patterns as well as the ability to predict the impact of treatment on both resting and dynamic expression is requisite.43 An example of patient evaluation and customized treatment can be found in Video 1 and examples of off-label treatment can be seen in Figures 9 and 10.

Figure 8. The muscles of the SMAS and the direction of pull for each group.
(Image courtesy of xMedica, LLC)
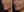
Figure 9. A 52-year-old female at baseline (A), and 6 weeks after treatment with 10 U of DAXI in the upper lip, divided evenly between two injection points on each side to produce eversion (B).
(Image courtesy of Sue Ellen Cox, MD, FAAD)

Figure 10. A 23-year-old female before (A, C) and 18 weeks after (B,D) treatment with DAXI. The patient received 40U of DAXI to the glabella,12U to each temporalis muscle, and 32U to the masseter muscle. The patient is shown at rest (A and B) and a maximum frown (C and D).
(Image courtesy of Rebecca Fitzgerald, MD)
Conclusion
Further experience will shed light on how DAXI performs in myriad clinical settings. Thus far, DAXI has been a welcome addition to the aesthetics treatment armamentarium as a potentially longer-lasting toxin with desirable diffusion properties that facilitate natural-looking results. As with all new technologies, our understanding of product behavior in different patient types, in combination with other modalities, and in off-label areas will continue to evolve. Ultimately, the current understanding, based on the science and clinical experience, suggests that all neurotoxins have the same mechanism of action, and differentiation of product performance is based primarily on the effective dose that reaches the neuromuscular junction. The concentration of toxin present at the junction seems to be dictated, in part, by unique excipients and lack thereof. Diligent observation of results in the first patients treated with DAXI will facilitate a better understanding of product action and permit fine-tuning of results. Optimum patient selection and injection techniques will deliver the best results for our patients with this exciting new BoNTA formulation.
References
1. Daxxify [prescribing information]. Newark, CA: Revance Therapeutics, Inc.; September 2022.
2. Carruthers JD, Fagien S, Joseph JH, et al. DaxibotulinumtoxinA for injection for the treatment of glabellar lines: Results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg. Jan 2020;145(1):45-58. doi:10.1097/PRS.0000000000006327
3. Solish N, Carruthers J, Kaufman J, Rubio RG, Gross TM, Gallagher CJ. Overview of daxibotulinumtoxinA for injection: a novel formulation of botulinum toxin type A. Drugs. Dec 2021;81(18):2091-2101. doi:10.1007/s40265-021-01631-w
4. Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol. Aug 2014;12(8):535-49. doi:10.1038/nrmicro3295
5. Jabbari B. History of botulinum toxin treatment in movement disorders. Tremor Other Hyperkinet Mov (N Y). 2016;6:394. doi:10.7916/D81836S1
6. Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. Jan 1992;18(1):17-21. doi:10.1111/j.1524-4725.1992.tb03295.x
7. Datta Gupta A, Visvanathan R, Cameron I, Koblar SA, Howell S, Wilson D. Efficacy of botulinum toxin in modifying spasticity to improve walking and quality of life in post-stroke lower limb spasticity - a randomized double-blind placebo controlled study. BMC Neurol. May 11 2019;19(1):96. doi:10.1186/s12883-019-1325-3
8. Carruthers A, Kane MA, Flynn TC, et al. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine--a global, evidence-based botulinum toxin consensus education initiative: part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg. Mar 2013;39(3 Pt 2):493-509. doi:10.1111/dsu.12147
9. Fagien S. Botulinum toxin type A for facial aesthetic enhancement: role in facial shaping. Plast Reconstr Surg. Oct 2003;112(5 Suppl):6S-18S; discussion 19S-20S. doi:10.1097/01.PRS.0000082189.88054.35
10. Colasante C, Rossetto O, Morbiato L, Pirazzini M, Molgo J, Montecucco C. Botulinum neurotoxin type A is internalized and translocated from small synaptic vesicles at the neuromuscular junction. Mol Neurobiol. Aug 2013;48(1):120-7. doi:10.1007/s12035-013-8423-9
11. Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. Mar 2015;15(1):1-9. doi:10.1007/s40268-014-0077-1
12. Frevert J. Content of botulinum neurotoxin in Botox(R)/Vistabel(R), Dysport(R)/Azzalure(R), and Xeomin(R)/Bocouture(R). Drugs R D. 2010;10(2):67-73. doi:10.2165/11584780-000000000-00000
13. Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. Apr 2017;69(2):200-235. doi:10.1124/pr.116.012658
14. Field M, Splevins A, Picaut P, et al. AbobotulinumtoxinA (Dysport®), onabotulinumtoxinA (Botox®), and incobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins (Basel). 2018;10(12):535.
15. Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227-41. doi:10.2147/BTT.S65603
16. US Food and Drug Administration. Information for healthcare professionals: onabotulinumtoxinA (marketed as Botox/Botox Cosmetic), abobotulinumtoxinA (marketed as Dysport) and rimabotulinumtoxinB (marketed as Myobloc). Accessed February 20, 2023, https://wayback.archive-it.org/7993/20170112032330/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174949.htm
17. Botox [prescribing information]. Madison, NJ: Allergan; July 2020.
18. Bertucci V, Humphrey S, Carruthers J, et al. Comparing injectable daxibotulinumtoxinA and onabotulinumtoxinA in moderate and severe glabellar lines: additional analyses from a phase 2, randomized, dose-ranging, double-blind, multicenter study. Dermatol Surg. Dec 2017;43 Suppl 3:S262-S273. doi:10.1097/DSS.0000000000001364
19. Dineen SS, Bradshaw M, Johnson EA. Neurotoxin gene clusters in Clostridium botulinum type A strains: sequence comparison and evolutionary implications. Curr Microbiol. May 2003;46(5):345-52. doi:10.1007/s00284-002-3851-1
20. Benefield DA, Dessain SK, Shine N, Ohi MD, Lacy DB. Molecular assembly of botulinum neurotoxin progenitor complexes. Proc Natl Acad Sci U S A. Apr 2 2013;110(14):5630-5. doi:10.1073/pnas.1222139110
21. Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. Mar 15 2011;57(4):555-65. doi:10.1016/j.toxicon.2010.12.019
22. Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics. Dec 9 2010;4:325-32. doi:10.2147/BTT.S14902
23. Gu S, Rumpel S, Zhou J, et al. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science. Feb 24 2012;335(6071):977-81. doi:10.1126/science.1214270
24. Lee K, Zhong X, Gu S, et al. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science. Jun 20 2014;344(6190):1405-10. doi:10.1126/science.1253823
25. Kukreja R, Chang TW, Cai S, et al. Immunological characterization of the subunits of type A botulinum neurotoxin and different components of its associated proteins. Toxicon. May 2009;53(6):616-24. doi:10.1016/j.toxicon.2009.01.017
26. Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. Jul-Aug 2009;32(4):213-8. doi:10.1097/WNF.0b013e3181914d0a
27. Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. Apr 1 2012;26(2):e1-9. doi:10.2165/11599840-000000000-00000
28. Mele AR, Marino J, Dampier W, Wigdahl B, Nonnemacher MR. HIV-1 Tat length: comparative and functional considerations. Front Microbiol. 2020;11:444. doi:10.3389/fmicb.2020.00444
29. Yoon JS, Jung YT, Hong SK, et al. Characteristics of HIV-Tat protein transduction domain. J Microbiol. Dec 2004;42(4):328-35.
30. Leifert JA, Harkins S, Whitton JL. Full-length proteins attached to the HIV tat protein transduction domain are neither transduced between cells, nor exhibit enhanced immunogenicity. Gene Ther. Nov 2002;9(21):1422-8. doi:10.1038/sj.gt.3301819
31. Smyth T, Oliyai C, Joshi A. Stabilizing effect of RTP004 on nonspecific surface adsorption in drug product manufacturing of daxibotulinumtoxinA (DAXI). Toxicon. 12/01 2018;156:S105. doi:10.1016/j.toxicon.2018.11.253
32. Malmirchegini R, Too P, Oliyai C, Joshi A. Revance’s novel peptide excipient, RTP004, and its role in stabilizing daxibotulinumtoxinA (DAXI) against aggregation. Toxicon. 12/01 2018;156:S72-S73. doi:10.1016/j.toxicon.2018.11.174
33. Carruthers J, Solish N, Humphrey S, et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: a phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol Surg. Nov 2017;43(11):1321-1331. doi:10.1097/DSS.0000000000001206
34. Glogau R, Kane M, Beddingfield F, et al. OnabotulinumtoxinA: a meta-analysis of duration of effect in the treatment of glabellar lines. Dermatol Surg. Nov 2012;38(11):1794-803. doi:10.1111/j.1524-4725.2012.02582.x
35. Fabi SG, Carruthers J, Joseph J, et al. High-Dose Neuromodulators: A Roundtable on Making Sense of the Data in Real-World Clinical Practice. Aesthet Surg J Open Forum. Dec 2021;3(4):ojab036. doi:10.1093/asjof/ojab036
36. Rummel A. The long journey of botulinum neurotoxins into the synapse. Toxicon. Dec 1 2015;107(Pt A):9-24. doi:10.1016/j.toxicon.2015.09.009
37. Sipione S, Monyror J, Galleguillos D, Steinberg N, Kadam V. Gangliosides in the brain: physiology, pathophysiology and therapeutic applications. Front Neurosci. 2020;14:572965. doi:10.3389/fnins.2020.572965
38. Pulkoski-Gross MJ, Fang J, Shai R, Too P. A cell-penetrating peptide (CPP) binds directly to and enhances membrane binding of the core toxin of botulinum neurotoxin serotype A (BoNT/A). Toxicon. 2022;214(Suppl 1):S47.
39. Weisemann J, Rummel A, Oliyai C, Too PH-M, Joshi A. Novel peptide excipient RTP004 enhances the binding of botulinum neurotoxin type A cell binding domain HC to rat brain synaptosomes. Toxicon. 2018;156:S113. doi:10.1016/j.toxicon.2018.11.273
40. Bertucci V, Green JB, Fezza JP, Brown J, Gallagher CJ. Impact of glabellar injection technique with daxibotulinumtoxinA for injection on brow position. Aesthet Surg J. Nov 2 2022;doi:10.1093/asj/sjac002
41. Solish N, Kane MAC, Biesman BS, Brown J, Gallagher CJ. Impact of daxibotulinumtoxinA for injection on brow position and frontalis muscle activity following treatment of glabellar lines. Aesthet Surg J. Sep 12 2022;doi:10.1093/asj/sjab362
42. Monheit G, Lin X, Nelson D, Kane M. Consideration of muscle mass in glabellar line treatment with botulinum toxin type A. J Drugs Dermatol. Sep 2012;11(9):1041-5.
43. Abramo AC, Do Amaral TP, Lessio BP, De Lima GA. Anatomy of forehead, glabellar, nasal and orbital muscles, and their correlation with distinctive patterns of skin lines on the upper third of the face: reviewing concepts. Aesthetic Plast Surg. Dec 2016;40(6):962-971. doi:10.1007/s00266-016-0712-z
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!



